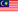FIELD CORRECTIVE ACTION LISTING FOR JUNE 2025
The list below contains Medical Device’s Field Corrective Action for the month of June 2025. This list provides field corrective action reporting information to MDA by establishment in accordance to requirement stipulated in Section 41, Act 737 and Regulation 6 of Medical Device (Duties and Obligations of Establishment) Regulations 2019.
Field Corrective Action (FCA) is part of a post-marketing risk assessment measure to ensure the continued safe use of medical devices in the Malaysia market. It is a corrective or preventive action in relation to a medical device which may include –
- The return of the medical device to the establishment;
- Modification of the medical device;
- Exchange of the medical device;
- Destruction of medical device; or
- Specific advice on the use of the medical device.
If you have experienced any safety and performance issue with one of the medical devices listed for FCA action, please seek advice from your medical device authorised representative as soon as possible.
|
No. |
Date Received |
Title of FCA |
Affected Medical Device |
MDA Reference Number |
MDA Registration Number |
Establishment Detail |
|
1. |
03/06/2025 |
ROTEM® SIGMA |
MDA/FCA/P1301-65308058-2025 |
IVDC10235825-198676 |
STRAITS SCIENTIFIC (M) SDN BHD
|
|
|
2. |
03/06/2025 |
ACL TOP FAMILY |
MDA/FCA/P1302-81066371-2025 |
IVDC8262823-138692 |
||
|
3. |
08/06/2025 |
MASSCHROM® AMINO ACIDS AND ACYLCARNITINES FROM DRIED BLOOD (NON DERIVATISED |
MDA/FCA/P1314-36051957-2025 |
IVDC8881820-44301 |
ANALISA RESOURCES (M) SDN BHD |
|
|
4. |
09/06/2025 |
ORTHO VISION ANALYZER SYSTEM |
MDA/FCA/P1315-72182972-2025 |
IVDD4853923-138156 |
T T MEDICAL MANAGEMENT SDN BHD |
|
|
5. |
09/06/2025 |
SPECTRAL CT |
MDA/FCA/P1315-33995668-2025 |
GC6307722-96412 |
PHILIPS MALAYSIA SDN BERHAD |
|
|
6. |
10/06/2025 |
ADVIA 2120I HEMATOLOGY SYSTEM |
MDA/FCA/P1319-85603243-2025 |
IVDB3655169817 |
SIEMENS HEALTHCARE SDN. BHD. |
|
|
7. |
11/06/2025 |
2024-IGT-BST-015 Philips Azurion Systems RI.x and R2.x Software issue potentially resulting in loss of imaging (X-ray) functionality
|
AZURION 3 |
MDA/FCA/P1318-19018758-2025 |
GC99381544218 |
PHILIPS MALAYSIA SDN BERHAD |
|
8. |
AZURION 5 |
MDA/FCA/P1320-13417835-2025 |
GC10785320-48186 |
|||
|
9. |
AZURION 7 |
MDA/FCA/P1321-82432683-2025 |
GC82521235717 |
|||
|
10. |
AZURION BIPLANE |
MDA/FCA/P1322-27795446-2025 |
GC59959884718 |
|||
|
11. |
13/06/2025 |
VITROS CHEMISTRY PRODUCTS ELECTROLYTES REAGENTS |
MDA/FCA/P1325-38709250-2025 |
IVDB7599223-142604 |
T T MEDICAL MANAGEMENT SDN BHD |
|
|
12. |
18/06/2025 |
2024-IGT-IGTD-006 Visions PV.018 Digital IVUS Catheter Instructions for Use (IFU) Precaution Update |
EAGLE EYE® PLATINUM DIGITAL IVUS CATHETER |
MDA/FCA/P1326-56147634-2025 |
GD772781341919 |
PHILIPS MALAYSIA SDN BERHAD |
|
13. |
19/06/2025 |
FA1469 SmartSync Software Update to Eliminate Potential Erroneous Electrical Reset Message |
CARELINK SMARTSYNC™ DEVICE MANAGER |
MDA/FCA/P1327-14153342-2025 |
GC2117624-178897 |
MEDTRONIC MALAYSIA SDN BHD |
|
14. |
19/06/2025 |
FA1475 SmartSync Software Update to Align Abort Button Behavior Across Programmer Platforms |
CARELINK SMARTSYNC™ DEVICE MANAGER |
MDA/FCA/P1328-36402143-2025 |
GC2117624-178897 |
|
|
15. |
24/06/2025 |
NELLCOR BEDSIDE SPO2 PATIENT MONITORING SYSTEM |
MDA/FCA/P1330-92315695-2025 |
GC64673492817 |
||
|
16. |
25/06/2025 |
AQUADIS 56 |
MDA/FCA/P1299-63848096-2025 |
GB3393023-131060 |
RBD HEALTHCARE SDN BHD |