THE TRAINING OF IMPORT AND EXPORT OF MEDICAL DEVICE REQUIREMENTS SESSION 2
Join us for the Training of Import and Export of Medical Device Requirements (Session 2) on 17 September 2025.
This comprehensive program offers in-depth knowledge and hands-on experience in managing regulatory processes under the Medical Device Act 2012 (Act 737).
Don’t miss the opportunity to strengthen your expertise and ensure compliance in the medical device trade.
Location: Cyberjaya/Putrajaya (TBC)
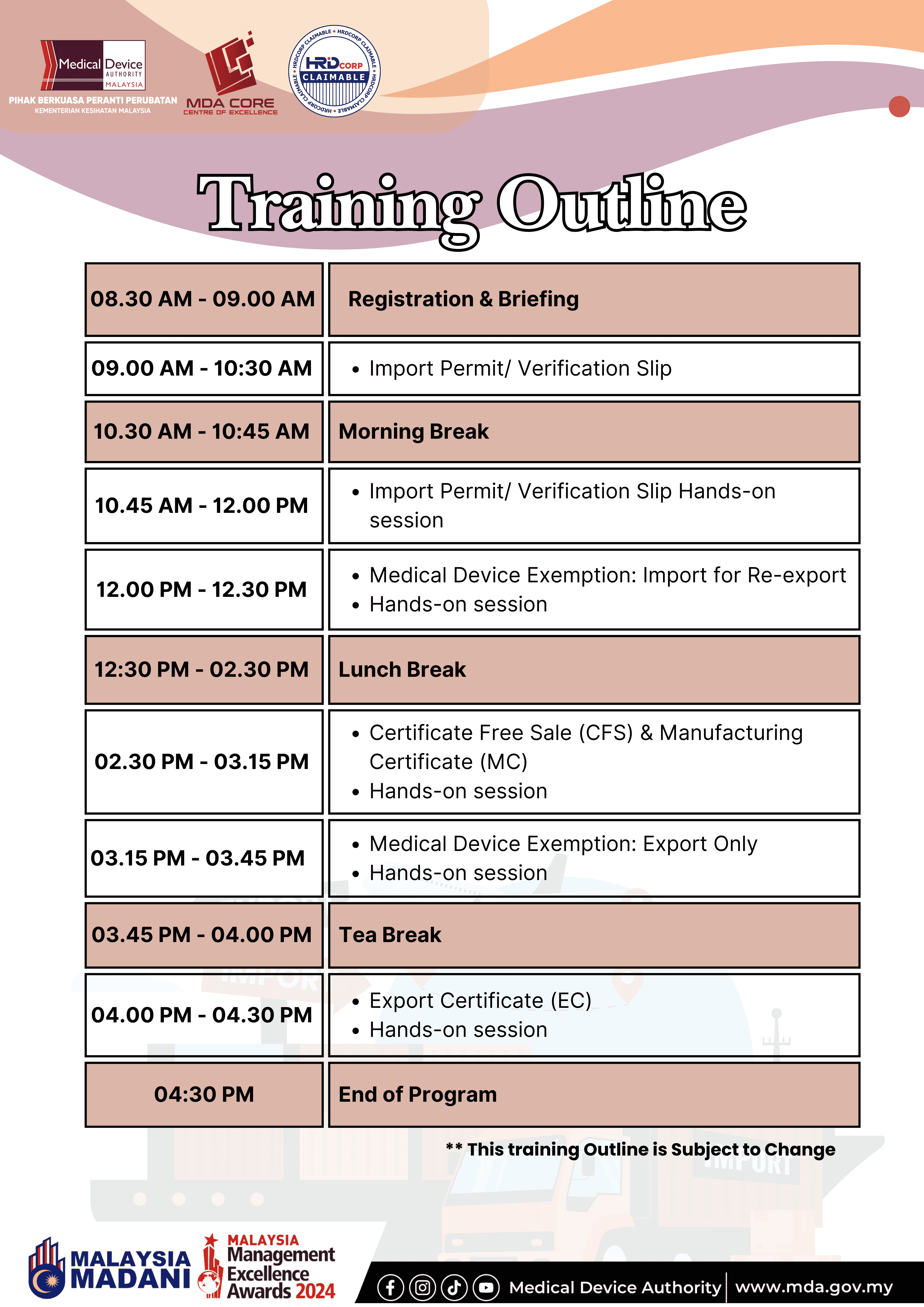
Time: 8.30 AM – 4.30 PM
Registration closes: 8 September 2025

Prepared by: MDA CORE (Administrative Division)
Uploaded by: Corporate Communication Division
Date of upload: 19 August 2025