CLINICAL RESEARCH STUDY
Definition : “clinical research” means any systematic investigation or study that determines the safety and effectiveness of medications, devices, diagnostic products, medical procedures and treatment regimens intended in or on one or more human subjects, in an adequate human clinical environment.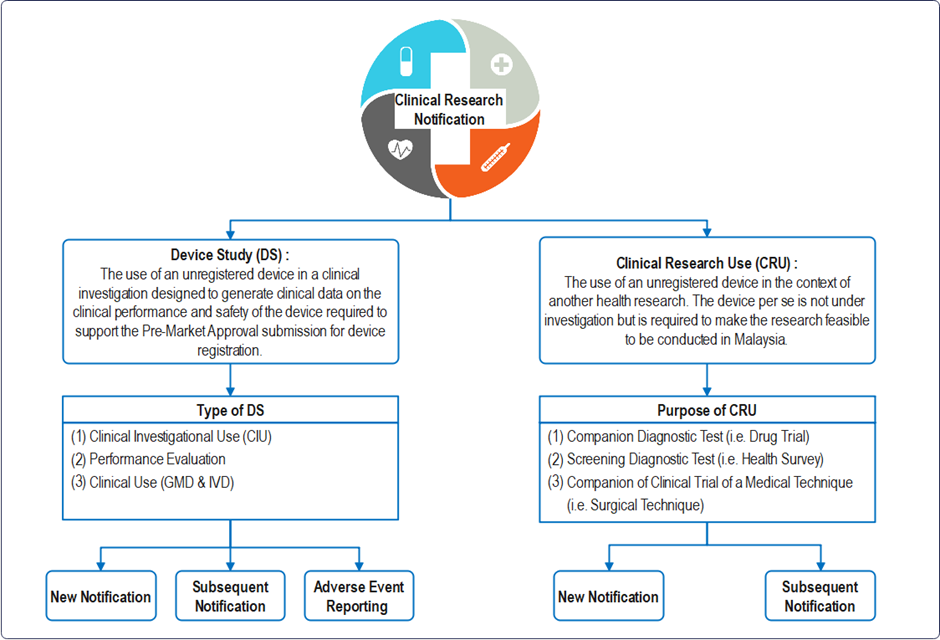
A. Link to online Notification Form : Medcast
B. Instant Guide to Create Medcast Account : Annex B Flow Chart
C. Instant Guide for Device Study (DS) Notification:
2. E-Submission Guide for New Notification
3. E-Submission Guide for Subsequent Notification
4. E-Submission Guide for Adverse Event Reporting
5. Technical Committee Meeting Calendar (TCMDCE)
6. Clinical Investigational Plan (CIP) - ISO14155
7. Clinical Performance Study Protocol (CPSP)
8. Investigational Brochure (IB) - ISO 14155
9. Refer Standard 14155
D. Instant Guide for Clinical Research Use (CRU) Notification:
1. Explanatory on Notification of Exemption for Clinical Research Use : CRU
2. Annex A : CRU Flow Chart
3. Annex C : E-Submission Guide for New Notification
4. Annex D : E-Submission Guide for Subsequent Notification
5. Annex E : Form Notification for Export /Disposal of Devices After Research Completion /Termination
E. Any inquiries, please email to: ci @ mda.gov.my
F. Contact number of the relevant officers:
Device Study :
1. Puan Rosmani +603-8230 0371
Clinical Research Use :
1. Puan Aidahwaty M. Olaybal +603-8230 0341
2. Puan Rosmani +603-8230 0371
Importance Notice :
None
Updated: 9 October 2025