NOTIFICATION FOR EXPORT ONLY MEDICAL DEVICE
Notification of Export Only Medical Device
1. Introduction
- The importation, exportation, or placement of a medical device in the Malaysia market requires the medical device to be registered under Medical Device Act 2012 (Act 737). However, under Medical Device (Exemption) Order 2024 state that medical devices for purpose of export only are exempted from registration requirements and shall make an application for an exemption to the Authority.
- The exportation of unregistered medical device is allowed once the Export Only Medical Device Exemption Letter is issued by the Authority.
2. Requirements for Notification of Export Only Medical Device
- Applicant shall submit a notification before exportation of the first shipment.
- The application Export Only Medical Device Exemption shall be submitted via google form and the required documents are as follows:
|
No |
Documents
|
|
1 |
Registrar of Companies (ROC) certificate of applicant |
|
2 |
|
|
3 |
|
|
4 |
(Please refer to Figure 2: Determination whether LOA is required or not for Export Only Exemption) |
|
5 |
A copy manufacturer’s QMS ISO 13485 certificate |
|
6 |
A copy of Brochure/Leaflet/Label/IFU that contain information on brief description and intended use. |
|
7 |
A copy of establishment license (If applicable) |
Note: *new requirements for Notification of Export Only Medical Device
3. Determination whether LOA is required or not for Notification of Export Only Medical Device?
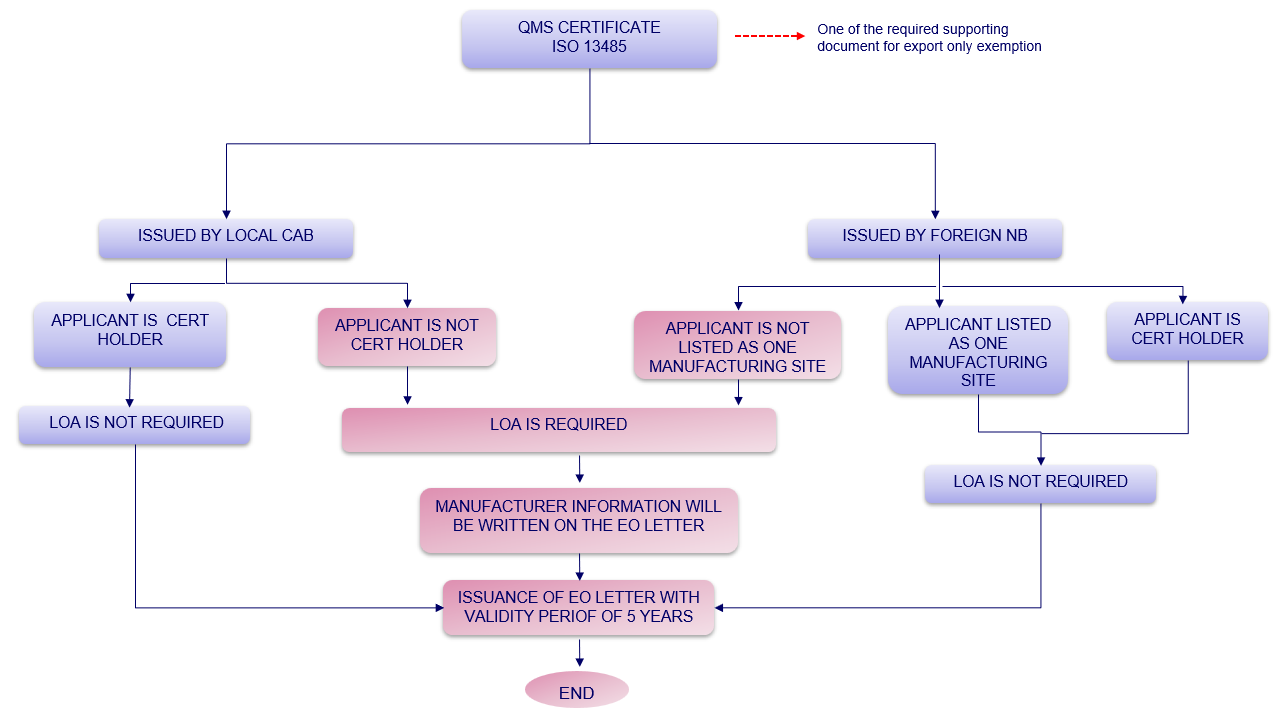
Figure 1: Determination whether LOA is required or not for Notification of Export Only Medical Device
4. Notification of Export Only Medical Device Process
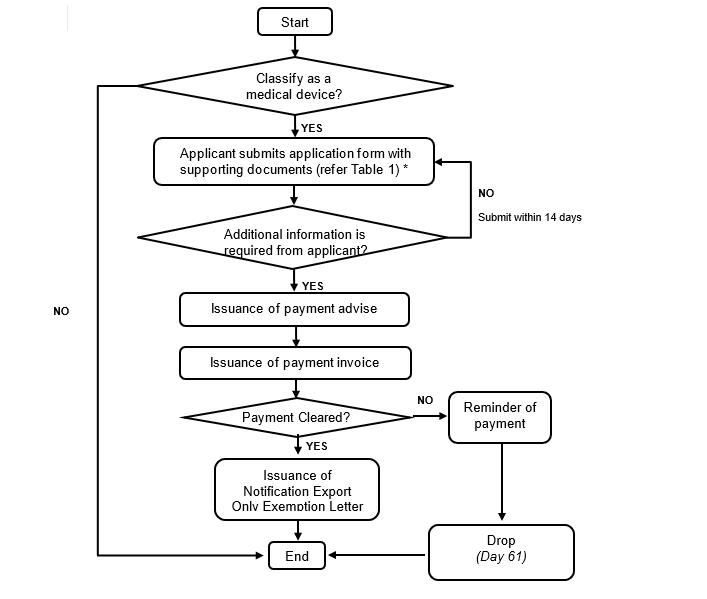
Figure 2: Notification of Export Only Medical Device Process
5. Fees for Notification of Export Only Medical Device
The payment RM500 shall be made online via portal BayarNow. For guidance on online payment is accessible via link or qr code below:

6. Issuance of Export Only Medical Device Exemption Letter
- Upon receipt of completed application and clearance of payment, the authority will issue an Export Only Medical Device Exemption Letter to the applicant within 14 days after clearance of payment, by letter and email.
- This Export Only Medical Device Exemption Letter permits multiple export consignments within the validity period of the letter.
- The validity period of the Export Only Medical Device Exemption Letter is five (5) years
- Once Export Only Medical Device Exemption Letter has expired or has been cancelled/withdrawn, no further export of the medical device, at any quantity, shall be permitted.
Any inquiries please email to exportonly.ec@mda.gov.my
General Line: +603 8230 0300
Direct Line : +603 8230 0253 (Pn. Nur Aisyah)
+603 8230 0208 (Pn. Hafizah)
Updated: 25 / 10 / 2023