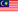REGISTRATION NOW OPEN! - DON’T MISS OUT ON SESSION 1 OF THE REGULATORY TRAINING POST-MARKET REQUIREMENTS
Greetings from the Medical Device Authority (MDA)!
We are pleased to announce that MDA will be conducting a Session 1 of the Regulatory Training Post-Market Requirements
The MDA Regulatory Training on Post-Market Requirements is a 1-day course designed to help participants understand their responsibilities under the Medical Device Act 2012 (Act 737) and related regulations.
This training covers key topics such as post-market surveillance, vigilance, incident reporting, and corrective actions, including recalls and field corrective actions (FCA). Participants will learn how to collect and analyse post-market data, identify reportable incidents, apply root cause analysis, and integrate these processes into their quality management system (QMS).
The session is ideal for manufacturers, authorized representatives, regulatory affairs managers, quality managers, medical device professionals, clinical staff, and anyone involved in maintaining compliance with post-market obligations in the medical device industry.
📅 Registration is now open until 1st August 2025.
🔗 Register here: https://forms.gle/zdqiHuYFf6QmPSd29 or scan the QR code from the poster.
💡 This training is claimable under the HRDCorp SBL Scheme, subject to HRDCorp’s terms and conditions.
For further inquiries, feel free to reach out to us at trainingpackage@mda.gov.my.
We look forward to your participation!
Thank you.


Prepared by : MDA CORE Unit
Uploaded by : Corporate Communication Division
Date of published : 14 July 2025